jelly alcohol is widely used in restaurants, hotels or in the home because the price of dry alcohol is quite affordable. The initial price is lower than that of other heat-generating products, especially dry alcohol consumption is safe and open and does not cause fire and explosion like mini gas cylinders.

The main raw materials are ethanol and methanol
Ethanol alcohol is the substance with the chemical formula C2H5OH. In folklore or called ethanol as alcohol. This is a colorless, non-toxic liquid with a special odor. Ethanol is the raw material used to produce agar alcohol because this is a clean, safe and cost-effective sample.
technology transfer of jelly alcohol production
Because Ethanol is made up of clean raw materials such as sugarcane, cassava, rice ... It is also called bio-raw material for this reason.
It is safe because when burned, Ethanol produces carbon dioxide and water.
These are 2 substances that are not harmful to human health. Ethanol's price is not too high because it is made up of materials in Vietnam that can be grown in large quantity, without having to import, so Ethanol's price is low, not too expensive.
Use methanol as a material to supply raw materials
Currently on the market share there are alcohol agar of unknown origin, instead of using ethanol, they use methanol as a material to supply alcohol agar.

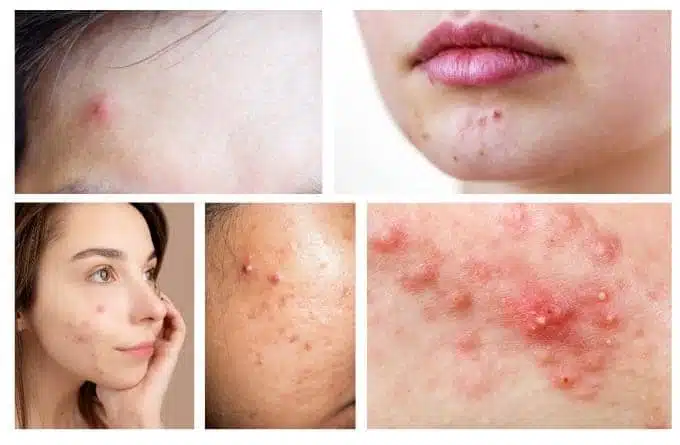
Methanol is a quite toxic chemical, when it burns methanol it produces smoke and toxic substances that when inhaled you will feel so uncomfortable that it seems impossible to breathe.
If this state is prolonged, you will suffer from respiratory distress. For example, when your eyes are in common contact with these toxic smoke streams, at first the eyes will feel burning, then eye inflammation leading to corneal tear. There is a very dangerous consequence to be found in the use of low-quality products that are at a hefty price tag.
Through here we realize that the main part of a substance plays a very important role, it determines its properties and applications in life.
Winter admixtures:
As you know, the order of alcohol production is based on the principle of freezing the alcohol solution obtained after the ethanol is melted and condensed.
To promote the coagulation of the alcohol solution after the condensation, a coagulant additive is used. This acts as a catalyst to accelerate the reaction to occur more quickly.
Coagulant additives play a very important role in the alcohol supply process.
For example, without the involvement of coagulant additives, the supply process cannot be successful.
Flame inhibitor:
To explain the role of this compound, I will use an example for you to easily imagine. When you cook the hot pot, the hot pot water is overflowing. At this time, the water overflows to the kitchen, followed by bubbles that will accumulate on the kitchen wall. These bubbles will dry and build up on the stove.
Fire in the kitchen will catch with dry air bubbles, which catch fire and create black smoke that causes a burning odor and stinging eyes, a very unpleasant sensation. If you do not bring the additive to prevent fire, the above phenomenon will occur and you will feel extremely annoyed and also very toxic.
The water in the tincture distribution formula plays two roles. One is to act as a solvent to dissolve the original mixture so that the substances are mixed in a uniform way, ensuring high quality products. The second role of water in the formula is that they heat the water to heat it up. Use the heat of the kettle that has just been heated to melt it properly, in which the alcohol vapor rises and condenses.
One, the secret to preparing dry alcohol with consumption of saturated calcium acetate
When calcium acetate is saturated in aqueous solution with alcohol, it will form dry alcohol in the form of calcium acetate colloidal.
The above results can be explained by the "solvent replacement method":
-
when the current state parameter changes, the congeners existing in the dispersed medium become larger in equilibrium, thus of the process will take place in the direction of transition back to equilibrium, is a new phase is created.
In this trick the solvent is replaced, that is, changing the composition of the medium.
-
Therefore, calcium acetate is saturated in water, but it becomes too saturated in alcohol-water (calcium acetate is insoluble in alcohol) so condensation will occur.
-
In this way dry alcohol is prepared from 75 ml of ethyl alcohol (ethanol) and 10 ml of saturated calcium acetate (prepared from 3g of calcium acetate and 10 ml of water) 17 respectively possessing a ratio of 7.5: 1.
-
And from 40 ml of ethanol and 10 ml of calcium acetate saturated.
Second, the preparation of dry alcohol uses fatty acids and alkalis
2.1 In hot alcohols, fatty acids are lower dissolved and rapidly alkaline forming a gel-forming agent, fatty acid soap and water.
C17H35COOH + NaOH -----> C17H35COONa + H2O
The sodium stearate formed will partially dissolve in water and form a hard crust. The alcohol then seeps into this hard crust and forms dry alcohol
2.2 Products

=> The obtained products have the following advantages:
Clean burning product, does not possess soot.
Smokeless, odorless and visible at fire.
In the composition of the dry alcohol fuel this gel can also be mixed with certain substances without having an adverse effect on the properties of the product.
Such substances may be:
-
dyes (such as Phenolphtalein, Rose Bengal) used to indicate or to add to the aesthetic value of products;
-
substances used to color the flame such as sodium salts and potassium salts of nitrates and chlorates, as well as salts of Li, Bo, Cu...
-
These ingredients can only be consumed in small amounts, usually not exceeding 1. % by weight and most suitable is 0.5%.
3, the preparation of dry alcohol uses a cellulose derivative that prevents hydration.
In the pH area of the solution is small, the ion groups are ionized to interact, causing the molecular circuit to shrink somewhat, so the viscosity also decreases.
When the pH of the solution is raised, the molecular circuit of the electrolytic polymers expands due to the increased ionization, so the viscosity of the solution also increases.
It turns out that when mixing the water and alcohol components, Hydroxypropyl methyl cellulose is practiced by lowering the pH of the mixture cheaply.
Then the pH of the pot is increased, increasing the viscosity and the alcohol is converted to a gel.
(1): firstly mix 200 ml of alcohol with 50 ml of water.
(2): Then 10g of Methocel J75 MS is added, obtained a viscous solution with water.
(3): Pour this thick solution into an existing container with enough alkali to raise the pH to 8 or above 8 (the amount of sodium hydroxide used is 2 to 4 grams). Gel formation will occur immediately.
possessing cellulose derivative with a hydration barrier layer
Dry alcohol fuels prepared in this manner include:
-
10g Methocel J75 MS
-
50g of water
-
170g of wine
-
2-4g NaOH
And very small amounts of alumin trihydrate are added to suppress
=> Made product possesses characteristics such as:
- Avoid generating toxic ingredients when burning.
Safe and easy to transport and use
4, how to prepare dry alcohol using inorganic fuel
4.1 - Add 72 g of silicon dioxide and 1,2 g of calcium hydroxide to 2 874 g of ethanol and stir rapidly for about 1.5 minutes.
- Then add 54g Hydroxypropyl cellulose and mix well. This pot will solidify gradually until it looks like a homogeneous gel block (about 1.5 hours).
4.2 - Add 6g of silicon dioxide, 6g of titanium dioxide and 0.1g of calcium hydroxide to 2898g
- Then add 90g Hydroxypropyl cellulose and mix well.
4.3 - Add 6g of silicon dioxide, 6g of aluminum oxide to a 026g of ethanol.
- Add 84g of Metylhydroxybutyl and mix for about 10 minutes (with speed
Then add 374g of water.
- After 20 minutes, add a 504g of Ethanol to obtain a homogeneous gel block.
In order to have 100% alcohol, you must purchase a water-free method of alcohol solution. There is a 90% alcohol sample on the market share. To separate water, it is purely to use chemical methods with chemicals available. The simplest method is to use anhydrous copper sulfate salt (white crystals) to absorb water carried in alcohol. Conduct the water water extensively to remove the water in the alcohol thoroughly.
In terms of structure, dry alcohol is made from ethyl alcohol and other additives. If done according to this recipe, dry alcohol is actually an effective and safe fuel.
But now for economic purposes, some people ignore the health of people who mix methyl alcohol to distribute dry alcohol.
Because the price of ethyl alcohol is better than the lake compared to ethyl alcohol, and mainly Dry alcohol without label, unknown original reason.
Because when burned up, methyllic will be burned less universally, which reduces the risk of toxicity to the body. The part that does not burn off is relatively new is the main cause of harm to the body.
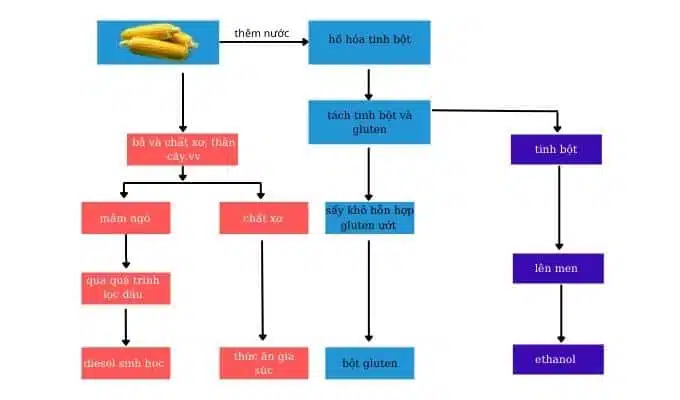
soure: https://quangtrungchem.com.vn/nha-may-san-xuat-ethanol-tu-ngo.html